Professor Myung Jae-ha's research team at Incheon National University presents an international academic paper on the development of flexible solid oxide fuel cells and strategies to overcome ceramic brittleness
- 글번호
- 405482
- 작성일
- 2025-04-21
- 수정일
- 2025-04-21
- 작성자
- 홍보팀 (032-835-9490)
- 조회수
- 615

Incheon National University (President Park Jong-tae) announced that Won Bo-ram, a Ph.D. student (1 author) and Myung Jae-ha, a corresponding author, published the results of the development of solid oxide fuel cells (SOFC) that overcome the brittleness limit of existing ceramic fuel cells by controlling the crystal structure of zirconia in the international journal Chemical Engineering Journal.
Solid oxide batteries are ceramic-based high-temperature electrochemical devices that can produce H2 and synthetic fuels as well as high-efficiency and pollution-free power generation without precious metal catalysts, but due to the brittleness of existing ceramic electrolytes, they are limited to large-scale political power generation devices.
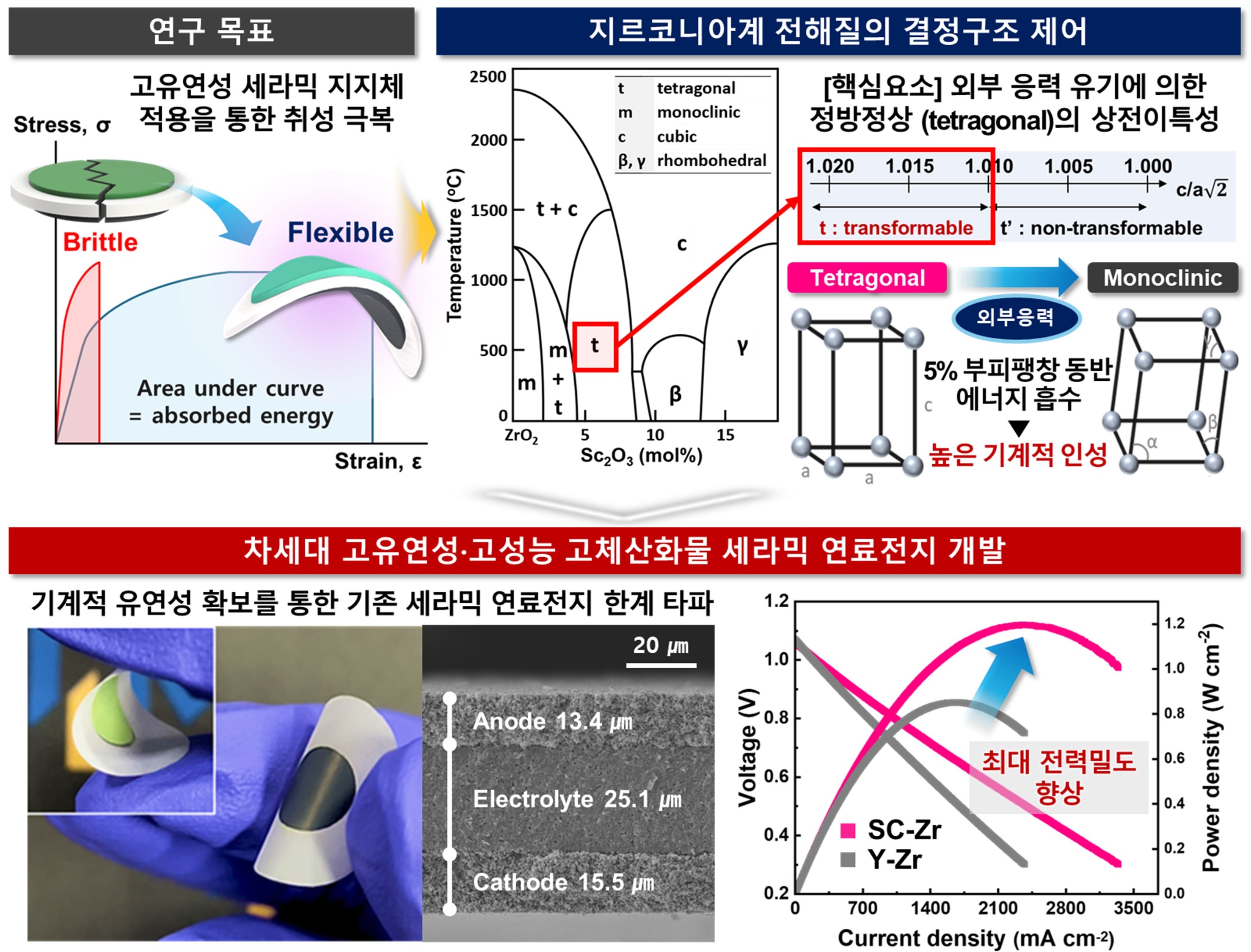
In order to overcome the brittleness of these solid oxide batteries, the research team conducted a study on the development of "flexible solid oxide batteries" and controlled the crystal structure and phase stability of tetragonal zirconia to demonstrate a very flexible Scandia stabilized zirconia (Sc2O3-ZrO2) system and its mechanism.
On the other hand, the core element for realizing a highly flexible ceramic electrolyte is transformable (transformable) square-phase zirconia, which causes phase transition to a monoclinic phase when mechanical stress is applied, entails volume expansion of up to 5%, and has properties that prevent mechanical destruction of ceramic materials. The phase transition characteristics of this square-phase external organic stress impart excellent mechanical toughness and flexibility to thin ceramic electrolytes. In this study, the amount of scandia doping was controlled to control the crystal structure of zirconia to a tetragonality range (c/a, >1.010) corresponding to the deformable square-phase.
The Scandia-stabilized zirconia-based highly flexible electrolyte developed in this study showed excellent elasticity and durability despite repeated bending more than hundreds of times, and finally implemented a highly flexible electrolyte-supported solid oxide unit cell (cell thickness <55 µm) using simple techniques such as tape casting and screen printing.
The unit cell made of a highly flexible new electrolyte material recorded a maximum power density of 1.2 W/cm2 in the hydrogen fuel cell mode at 900°C and proved its excellent durability by operating stably without deterioration even after 350 hours of continuous driving. In addition, the highest power density among the flexible SOFCs reported at all temperatures (700–900°C) was achieved, and the cell performance was significantly improved by more than 123% at 700°C compared to the conventional flexible yttria stabilization (Y2O3-ZrO2) electrolyte.
The research team's highly flexible solid oxide fuel cell (SOFC) proved its potential as a key technology that improves battery performance and enables light weight and miniaturization of the system by minimizing the ion conduction distance of the electrolyte by drastically reducing the thickness of the electrolyte by more than 8 times compared to the existing commercial electrolyte-supported SOFC (~200 μm) based on the excellent strength and moldability of the electrolyte.
Therefore, this technology shows the applicability of SOFC in a variety of fields, including small devices and mobile devices, as well as for large-scale power generation, and is expected to contribute to IMO regulation and hydrogen society as a core technology for transportation fuel cells such as hydrogen ships, hydrogen trucks, and aircraft.
This study was conducted with the support of the Korea Research Foundation (Excellent Research) and the Korea Institute of Industrial Technology Planning and Evaluation with the funding of the Ministry of Science and ICT and the Ministry of Trade, Industry and Energy, and was published in March in the Chemical Engineering Journal (IF=13.4).
- 첨부파일
- 첨부파일이(가) 없습니다.

